Introduction
In the world of modern biopharmaceuticals, few cell lines have had as profound an impact as the Chinese Hamster Ovary (CHO) cell line. From therapeutic antibodies to recombinant enzymes, CHO cells have become the workhorse of the biotechnology industry. One of the most vital classes of therapeutics produced using these cells are clotting factors – crucial proteins responsible for the regulation of blood coagulation. These recombinant clotting factors have transformed the treatment landscape for individuals suffering from bleeding disorders such as hemophilia A and B, providing safer and more effective alternatives to plasma-derived products.
Definition
Chinese Hamster Ovary (CHO) clotting factors are blood coagulation proteins that are produced using Chinese Hamster Ovary cells through recombinant DNA technology. CHO cells serve as a widely used mammalian expression system because they can perform complex post-translational modifications, such as proper folding and glycosylation, ensuring the clotting factors closely resemble their natural human counterparts. These recombinant clotting factors are commonly used in the treatment of bleeding disorders like hemophilia, offering a safe and consistent alternative to plasma-derived products.
The Importance of Clotting Factors in Human Physiology
Blood clotting, or coagulation, is an intricate cascade involving over a dozen specialized proteins that interact in a tightly regulated sequence. When a blood vessel is injured, the clotting cascade ensures that fibrin – a strong, fibrous protein – forms to stop bleeding. Two of the most clinically relevant clotting factors are Factor VIII (FVIII) and Factor IX (FIX). Deficiencies or dysfunction in these factors lead to hemophilia A and hemophilia B, respectively.
Historically, treatment relied on plasma-derived clotting factors, isolated from donated human blood. While effective, these therapies carried risks, including viral transmission (notably HIV and hepatitis C in the 1980s) and supply limitations. The advent of recombinant DNA technology revolutionized this field, allowing scientists to clone the genes encoding clotting factors and express them in cultured mammalian cells – most successfully, CHO cells.
Why CHO Cells?
The choice of CHO cells is not arbitrary. Since their introduction in the 1950s, CHO cells have proven uniquely suited for industrial protein production for several reasons:
- Post-Translational Modifications (PTMs): Clotting factors are heavily glycosylated proteins that require specific post-translational modifications, such as γ-carboxylation and sulfation, for biological activity. CHO cells possess the machinery to perform these modifications in a way that closely mimics human cells.
- Genetic Stability and Adaptability: CHO cells can be easily genetically engineered and adapted to serum-free, suspension culture systems, making them ideal for high-density, large-scale bioreactors.
- Regulatory Acceptance: Decades of safety data have made CHO-derived therapeutics highly trusted by regulatory agencies such as the FDA and EMA.
- Robust Productivity: CHO cells can produce large quantities of complex recombinant proteins without compromising quality, which is essential for cost-effective biopharmaceutical manufacturing.
These attributes have made CHO cells the default host for producing most of the world’s approved recombinant clotting factors.
CHO-Produced Clotting Factors: A Closer Look
Recombinant Factor VIII (rFVIII):
Factor VIII is a large glycoprotein that serves as a cofactor in the activation of Factor X, a key step in the coagulation cascade. Its production presents significant challenges due to its complex structure and extensive glycosylation.
CHO cells have successfully been used to produce several generations of rFVIII products:
- First-generation rFVIII (e.g., Recombinate®) used CHO cells cultured in media containing human serum albumin.
- Second-generation rFVIII eliminated human-derived proteins from the production process but retained albumin as a stabilizer.
- Third-generation rFVIII, such as Advate® and Nuwiq®, are entirely human- and animal-protein-free, relying solely on CHO or human cell expression systems and synthetic stabilizers.
These advancements significantly improved product safety, purity, and consistency.
Recombinant Factor IX (rFIX):
Factor IX, essential for the activation of Factor X, is a smaller and somewhat simpler protein than FVIII, but it requires γ-carboxylation for biological activity – a modification dependent on vitamin K metabolism. CHO cells can perform this modification efficiently when supplemented with appropriate cofactors.
Recombinant FIX products, such as BeneFIX® and Alprolix®, are CHO-derived and provide reliable, long-acting treatment options for patients with hemophilia B. The success of these therapies further demonstrates the versatility of CHO cells in expressing proteins with intricate modification requirements.
Engineering CHO Cells for Improved Clotting Factor Expression
While CHO cells are powerful, producing clotting factors – especially FVIII – is no small feat. These proteins are large, prone to aggregation, and often expressed at low levels. To overcome these challenges, researchers employ advanced cell line engineering strategies:
- Gene Amplification and Promoter Optimization: Strong promoters (e.g., CMV or EF1α) are used to drive high expression, and selection markers like DHFR or GS systems enable gene amplification.
- Codon Optimization: Altering the DNA sequence to favor codons preferred by CHO cells enhances translation efficiency.
- Chaperone Co-expression: Co-expression of molecular chaperones assists in proper folding and secretion of complex proteins like FVIII.
- Metabolic Engineering: Tweaking pathways involved in glycosylation, vitamin K metabolism, and energy production can enhance product yield and quality.
Recent developments in CRISPR/Cas9 genome editing have made it even easier to fine-tune CHO cell lines for maximal productivity and consistent post-translational modification profiles.
Downstream Processing and Quality Control
After production, recombinant clotting factors undergo extensive purification to ensure safety and efficacy. Downstream processes typically include:
- Affinity and Ion-Exchange Chromatography: To isolate the target protein and remove impurities.
- Virus Inactivation and Filtration: To ensure viral safety.
- Ultrafiltration/Diafiltration: To concentrate and formulate the final product.
Rigorous quality control testing ensures that the glycosylation patterns, activity levels, and purity meet strict regulatory standards. Because clotting factors are life-saving therapeutics administered to vulnerable patients, maintaining consistent quality is paramount.
The Impact on Hemophilia Treatment
CHO-derived clotting factors have completely transformed the standard of care for bleeding disorders. Patients who once faced frequent, risky transfusions now benefit from recombinant therapies with longer half-lives and fewer complications. Moreover, extended half-life (EHL) versions – achieved through PEGylation or Fc-fusion technologies – allow for less frequent dosing, improving patient adherence and quality of life.
For instance:
- Eloctate® (rFVIII-Fc fusion) and Alprolix® (rFIX-Fc fusion) are both produced in CHO cells.
- These engineered forms circulate longer in the bloodstream, reducing infusion frequency from multiple times per week to once every 7–10 days.
Future Trends of the Chinese Hamster Ovary (CHO) Clotting Factors Market
Advancements in Cell Line Engineering:
The next generation of CHO-based production will leverage CRISPR/Cas9 genome editing, synthetic biology, and AI-driven cell design to create hyper-productive, stable lines with optimized post-translational modifications for clotting factors like Factor VIII and IX.
Continuous and Automated Bioprocessing:
Manufacturers are shifting toward continuous manufacturing systems and real-time monitoring to improve yield consistency, reduce production costs, and ensure better scalability of recombinant clotting factor production.
Extended Half-Life and Novel Therapeutic Designs:
The market is witnessing rapid development of extended half-life (EHL) clotting factors and fusion proteins (e.g., Fc and PEGylated forms), improving dosing convenience and patient compliance.
Expanding Global Access and Biosimilars:
As patents expire, biosimilar clotting factors are entering the market, especially in emerging economies. This trend will democratize access to life-saving therapies while driving competitive pricing.
Integration of Humanized Expression Systems:
While CHO remains dominant, research into human-derived cell systems aims to complement CHO production by offering even more human-like glycosylation patterns, potentially improving therapeutic efficacy and reducing immunogenicity.
Growth Rate of Chinese Hamster Ovary (CHO) Clotting Factors Market
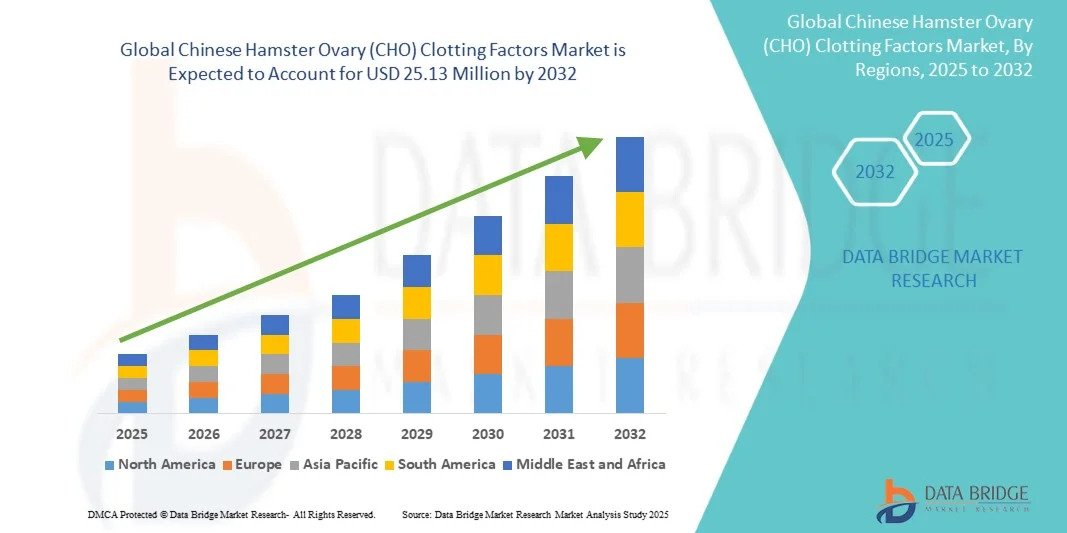
According to Data Bridge Market Research, the Chinese Hamster Ovary (CHO) clotting factors market was estimated to be worth USD 14.39 million in 2024 and is projected to grow at a compound annual growth rate (CAGR) of 7.22% to reach USD 25.13 million by 2032.
Learn More: https://www.databridgemarketresearch.com/reports/global-chinese-hamster-ovary-cho-clotting-factors-market
Conclusion
From a modest origin in the 1950s to their modern role as the powerhouse of biopharmaceutical production, CHO cells have fundamentally shaped the future of medicine. Their ability to produce complex, safe, and effective recombinant clotting factors has given millions of patients with hemophilia and related disorders access to life-saving therapies.

